In Depth Summary
Monomers to Polymers and Vice Versa
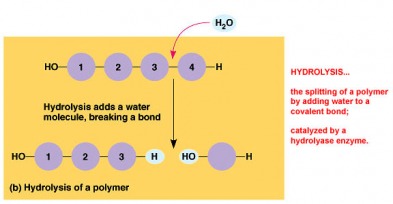
Macromolecules are giant organic molecules that fall into four categories: Carbohydrates, Lipids, Proteins, and Nucleic Acids. They are polymers that are built from monomers by a condensation or dehydration reaction which removes a water molecule to form a covalent bond. Hydrolysis is when the water molecule is re-added by enzymes to split the polymer back into individual monomers.
Carbohydrates
Carbohydrates are macromolecules assembled from single sugar molecules (the monomers) called monosaccharides. The bond between monosaccharides is a glycosidic bond. Disaccharides are two monosaccharides bonded by a glycosidic bond. Important disaccharides are…
· Glucose+Glucose=Maltose
· Glucose+Fructose=Sucrose
· Glucose+Galactose=Lactose
**Switch the ending of the disaccharide to –ase to form the name of the enzyme that degrades it (maltase, sucrose, and lactase)
Carbohydrates serve many important functions, including structure and fuel storage. Important polysaccharides in plants are starch and cellulose, while in animals there is glycogen and chitin. Starch consists of long chains of glucose monomers in the alpha configuration. The two main types are amylase (unbranched) and amylopectin (branched). Starch in plants is used to store fuel (glucose) and the starch itself is stored in structures called plastids (a family of organelles that includes chloroplasts, chromoplasts, and amyloplasts). Cellulose in plants is a touch structural polysaccharide formed from a chain of glucose molecules in the beta configuration and is the most abundant organic compound on Earth. Cellulose molecules cluster, about 80 together, to form a cellulose microfibril. These microfibrils are the major components in the cell wall. Glycogen in animals is more highly branched than amylopectin and is used for storage. It is formed of glucose monomers and found in liver and muscle cells. It is hydrolyzed when fuel is needed. Chitin is a polysaccharide similar to cellulose except that it has a nitrogenous appendage on each glucose monomer. It is actually leathery but becomes hardened when encrusted with calcium carbonate. Arthropods us it in their exoskeletons and fungi use it in their cell walls.
Lipids
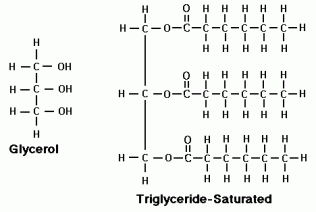
Lipids are very diverse hydrophobic macromolecules that fall under three main classes: fats, phospholipids, and steroids. Fats are not polymers because they are made of two types of molecules, glycerol and fatty acids, which are bound by ester linkages. Glycerol is an alcohol with 3 hydroxyl-group-bearing carbon atoms. Fatty acids have a carboxyl group followed by a long hydrocarbon chain, they can be saturated or unsaturated. Saturated fatty acids are “saturated” with hydrogen and each carbon is bound to 2 or more hydrogen atoms. This results in fats that easily stack together to be solid at room temperature. Unsaturated fatty acids have spots where carbon atoms double bond with each other, causing kinks in the fatty acid. This results in fats that don’t stack easily and are not solid at room temperature. Fats mainly function in energy storage (more than twice as much energy as a polysaccharide) and cushioning of organs and are stored in adipose cells. A phospholipid has a glycerol bonded to a phosphate group and two fatty acids, resulting in a hydrophilic head and hydrophobic tails (an ambivalent molecule). They form a lipid bilayer, with the hydrophobic tails facing inward and the hydrophilic heads facing outside, which results in the major component of our cell membrane. Steroids have a carbon skeleton of four fused rings. Cholesterol is a steroid that keeps the plasma membrane fluid, and many hormone of the endocrine system are also steroids.
Proteins
Proteins account for 50% of the dry mass of a cell and are involved in virtually all cellular activities. The main types of proteins are…
· Enzymatic proteins speed up chemical reactions
· Structural proteins support
· Storage proteins store amino acids
· Transport proteins transport other substances
· Hormonal proteins coordinate Organismal activities
· Receptor proteins cellular response to stimuli
· Contractile/Motor proteins create movement
· Defensive proteins protect against disease
The monomer of a protein is an amino acid. An amino acid consists of an alpha carbon surrounded by an amino group, a hydrogen atom, a carboxyl group, and the R group. The R group of an amino acid is its distinguishing characteristic and can make an amino acid non-polar, polar, acidic, or basic. Amino acids are then bound together by peptide bonds to from polypeptides. There are four levels of protein structure: primary, secondary, tertiary, quaternary. Primary structure of a protein is its unique sequence of amino acids. Secondary structure of a protein is when hydrogen bonds effect protein structure, causing the polypeptide to form alpha helixes and beta pleated sheets. Alpha helixes are delicate spirals help together by hydrogen bonds between every fourth amino acid. Beta sheets are when two parts of a polypeptide are parallel and form a pleated sheet. Tertiary structure is when the protein folds at an even higher level thanks to the action of hydrogen bonds, disulfide bridges, ionic bonds, and van der Waals interactions. Quaternary structure is the final structure of the protein, resulting from the association of different polypeptides. Sickle cell anemia is a mistake in the primary structure of hemoglobin causing a deformed version of the protein with severely limited oxygen carrying ability. Chaperone proteins help proteins fold by providing a hydrophilic environment. X-Ray Crystallography is a method used by scientists to find the 3D structure of a protein.
Nucleic Acids
Nucleic Acids are the group of macromolecules that code for our genetic information and are read to make proteins. The monomer of nucleic acids is the nucleotide. A nucleotide consists of a five carbon sugar, a phosphate group, and a nitrogenous base (either cytosine, thymine/uracil, guanine, or adenine). Thymine/Uracil and cytosine are the pyrimidines. Guanine and adenines are the purines. However, Guanine bonds to Cytosine and Thymine/Uracil binds to Adenine. There are two types polynucleotides, DNA and RNA. There are three main differences between DNA and RNA. DNA has deoxyribose for a sugar and RNA has ribose. DNA is double-stranded in a double helix, RNA is single stranded. DNA has Thymine as a complementary base to Adenine while RNA has Uracil to replace Thymine. In a cell, DNA is transcribed into mRNA. The mRNA exits the nucleus and goes to the ribosome to be read. The ribosome uses amino acids from tRNA to build the proper protein, thus DNA controls the cell.
The Link Below Is To Our Powerpoint
| molecules_of_life.pptx | |
| File Size: | 3849 kb |
| File Type: | pptx |
Key Terms

· Adipose Cells-fat storing cells in animals
· Alpha Carbon-the asymmetrical carbon in the middle of an amino acid
· Alpha Helix-a delicate spiral formed by hydrogen bonds between every fourth amino acid
· Ambivalent-a type of molecule that both attracts and repels water at different areas (phospholipid)
· Amino Acids-monomer of a protein
· Amylopectin-branched starch
· Amylose-unbranched starch
· Antiparallel-the way that the phosphate backbones of DNA twist around each other but never touch
· Atherosclerosis-a disease in which a lipid plaque clogs the arteries
· Beta Pleated Sheet-parallel sections of polypeptide forming hydrogen bonds to form pleated sheets
· Carbohydrates-sugars and their polymers that function in structure and storage (they are fuel)
· Catalysts-molecules that lower the activation for a chemical reaction
· Cellulose-structural polysaccharide in plants, found in cell walls
· Chaperonins/Chaperone Proteins-proteins that create hydrophilic to help polypeptides fold
· Chitin-structural polysaccharide in animals; cell walls of fungi, and arthropod exoskeletons
· Cholesterol-a steroid that keeps the plasma membrane fluid
· Cis Double Bond-a double bond carbon atoms in an unsaturated fatty acid· Condensation/Dehydration Reaction-covalent bonding of monomers by the removal of a water molecule
· C-Terminus-the carboxyl end of a polypeptide
· Denaturation-the unfolding of a protein by heat, pH, or other factors
· Deoxyribonucleic Acid (DNA)-doubled stranded nucleic acid that codes for proteins
· Deoxyribose-Pentose in DNA nucleotides
· Disaccharides-molecules made of two covalently bonded monosaccharides
· Disulfide Bridges-covalent bonds between sulfhydryl groups of amino acids
· Double Helix-shape of DNA
· Enzymes-protein catalysts that speed up a reaction without being changed themselves
· Ester Linkage-a covalent bond between a glycerol and fatty acid
· Exoskeleton-the hard outer skeleton of arthropods, composed of chitin
· Fat-lipids composed of glycerol and fatty acids
· Fatty Acids-a long hydrocarbon chain· Gene-a segment of DNA that codes for one protein
· Glycerol-an alcohol with 3 hydroxyl group bearing carbons
· Glycogen-a storage polysaccharide in animals that is more branched amylopectin
· Glycosidic Linkage-covalent bond between monosaccharides
· Hexose-a six carbon sugar
· Hydrocarbons-molecules consisting completely of hydrogen and carbon
· Hydrolysis-enzymes adding a water molecule to break monomers apart
· Hydrophilic-having an affinity for water
· Hydrophobic Interaction-water pushes hydrophobic amino acids toward the center of the polypeptide
· Hydrophobic-having no affinity for water, they do not mix with it
· James Watson and Francis Crick-used the data of other scientists in order to create the 3D structure of DNA
· Lipids-diverse molecules that are all hydrophobic
· Macromolecule-giant, covalently bonded, organic molecules
· Monomers-building blocks of a polymer· Monosaccharides-single sugars (1:2:1 ratio of CHO)
· mRNA-RNA read by ribosome to make protein
· N-Terminus-the amino end of a polypeptide
· Nucleic Acids-macromolecules composed of nucleotides
· Nucleoside-the nucleotide without the phosphate group
· Nucleotide-the monomer of nucleic acids, made of a phosphate group, a pentose, and a nitrogenous base
· Pentose-a five carbon sugar
· Phospholipid-a lipid that has a phosphate head that attracts water, and hydrophobic tails that don’t
· Plastids-a family of organelles that store starch
· Polymer-a chain of covalently bonded molecules (similar molecules)
· Polynucleotides-chains of nucleotides
· Polypeptides-chains of amino acids covalently bonded
· Polysaccharides-chains of monosaccharides
· Primary Structure-the amino acid sequence of a polypeptide
· Protein-the final macromolecule, including polypeptides and their folding
· Purines-Adenine and Guanine
· Pyrimidines-Cytosine and Thymine/Uracil
· Quaternary Structure-the final structure of a protein, multiple polypeptides together
· R Group-the variably chain of an amino acid that hangs off the asymmetrical carbon
· Ribonucleic Acid (RNA)-single stranded, manufactured from DNA
· Ribose-Pentose in RNA nucleotides
· Rumen-the first compartment of a cow’s stomach where cellulose digesting bacteria are found
· Saturated Fatty Acid-a fatty acid in which each carbon is bound to at least two hydrogen atoms
· Secondary Structure-the polypeptide forms hydrogen bonds, resulting in alpha helixes and beta pleated sheets
· Starch-a polysaccharide that plants use to store glucose (when glucose is needed it is hydrolyzed off the end)
· Steroids-lipids with four fused carbon rings
· Tertiary Structure-the structure of a polypeptide now with folding caused by ionic bonds, hydrogen bonds, disulfide bridges, and hydrophobic interactions
· Trans Fat-unsaturated fats that have been hydrogenated, forming trans bonds
· Triacylglycerol/Triglyceride-a fat molecule (it consists of 3 fatty acid chains)
· Triose-a three carbon sugar
· Unsaturated Fatty Acid-a fatty acid in which there are double bonds between carbon atoms, causing it to be kinky
· Uracil-the nucleotide in RNA that take the place of thymine
· Van der Waals Forces- a bond between the hydrophobic molecules in the center of the polypeptide
· X Ray Crystallography-an important technique in determining the three dimensional structure of a protein
Greek Words
· Monos-single
· Sacchar-sugar
· Proteios-first place
· Polys-many
· Meris-part
· Hydro-water
· Lysis-break